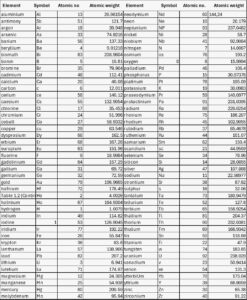
An atom is the smallest unit of an element that can participate in chemical reactions with other elements. Each element’s atom is represented by a symbol, usually consisting of the first letter or the first two letters of its English or Latin name. Elements, along with their symbols, atomic weights, and atomic numbers, are listed in reference tables such as Table 1.1. For instance, “O” is the symbol for an oxygen atom, whereas “O₂” denotes a molecule of oxygen. Similarly, “CO₂” represents a single molecule of carbon dioxide gas.
In the gaseous state, molecules of a substance are widely spaced and move rapidly in random directions. These molecules can be made up of a single atom, as in the case of helium (He), or consist of two or more atoms of the same element, such as hydrogen (H₂) or oxygen (O₂). Molecules of compounds, like water (H₂O) or carbon monoxide (CO), contain atoms of different elements.
When a gas condenses into a liquid, the molecules draw closer together. In the solid state, the atoms are arranged in a rigid structure, making it impossible to separate one group of atoms from the rest. A “molecule” is defined as the smallest possible particle of a compound that retains the fundamental combination of its atoms. Solid and liquid compounds are also represented by chemical formulas. For example, the mineral calcite is represented by the formula CaCO₃, indicating it is composed of calcium (Ca), carbon (C), and oxygen (O) in a ratio of one calcium atom, one carbon atom, and three oxygen atoms. A molecule of calcite is the smallest unit of the mineral that retains its atomic composition.
Atoms consist of three main subatomic particles: protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons carry a negative charge. Protons and neutrons have nearly the same mass, which is used as the standard unit of atomic mass. Together, they form the nucleus of the atom. In the case of hydrogen, the simplest element, the nucleus consists of just one proton. Electrons orbit the nucleus in numbers equal to the number of protons, so the positive charge of the protons is balanced by the negative charge of the electrons, rendering the atom electrically neutral. Since the mass of an electron is about 1/1850 of a proton or neutron, almost all the atom’s mass is concentrated in its nucleus.
| Nucleus electron | Proton | 1 positive charge |
| no charge | ||
| Electron | Neutron | 1 Negative charge |
- Proton (p⁺)
- Charge: Positive (+1)
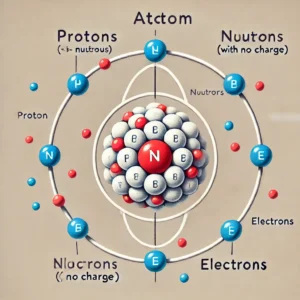
- Mass: Relatively heavy (about 1 atomic mass unit or amu)
- Location: Inside the nucleus of the atom
- Role: Protons determine the element of an atom. The number of protons in the
- The nucleus is called the atomic number and defines the chemical element. For example, hydrogen has 1 proton, helium has 2 protons, and so on.
- Neutron (n⁰)
- Charge: Neutral (0)
- Mass: Similar to that of a proton (about 1 amu)
- Location: Inside the nucleus of the atom
- Role: Neutrons help hold the nucleus together through the strong nuclear force. They also contribute to the mass of the atom but don’t affect its chemical identity.
- Electron (e⁻)
- Charge: Negative (-1)
- Mass: Very light (about 1/1836 the mass of a proton)
- Location: Orbiting the nucleus in regions called electron clouds or energy levels
- Role: Electrons are involved in chemical reactions and bonding. The arrangement of electrons in an atom determines its chemical behavior.
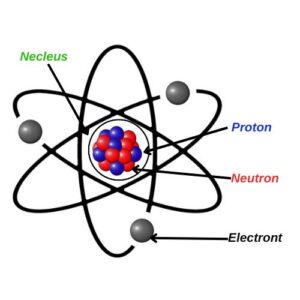
Structure of an Atom
- The nucleus contains protons and neutrons, which account for most of the atom’s mass.
- Electrons move around the nucleus in various energy levels or shells, creating the electron cloud.
In the Rutherford-Bohr theory of atomic structure, the electrons are pictured as revolving in fixed orbits around the nucleus, rather in the way that the planets revolve around the Sun. Although this picture is nowadays far too simple and inaccurate (with mathematical functions that express the probability of finding electrons in specific places), the Rutherford-Bohr picture is, nevertheless, a suitable model for dealing with the majority of elements dealt with in this elementary book.