The first chapter of the 11th Class Science curriculum covers five key points outlined below.
1.1 What is Physics
1.2 Scope and excitement of physics
1.3 Physics, Technology, and Society
1.4 Fundamental Forces in Nature
1.5 Nature of Physics laws
1.1 WHAT IS PHYSICS?
Humans have always been curious about the world around them. For as long as we can remember, the night sky with its shining celestial bodies has captivated our imagination. The daily cycle of day and night, the changing seasons, eclipses, tides, volcanoes, and rainbows have all been sources of wonder. The world presents an incredible variety of materials and a rich diversity of life and behavior. In response to the awe and mystery of nature, humans have observed the environment carefully, seeking patterns and connections in natural phenomena, and creating tools to engage with the world around them. Over time, this effort gave rise to modern science and technology.
The word “Science” comes from the Latin term scientia, meaning “to know.” Similar meanings are found in the Sanskrit word vijnan and the Arabic word ilm, both signifying knowledge. Science, in its broadest sense, is as old as humanity itself. Early civilizations such as Egypt, India, China, Greece, and Mesopotamia made significant contributions to its development. From the 16th century onward, science advanced rapidly in Europe, and by the mid-20th century, it had become a global endeavor with many cultures and nations contributing to its swift progress.
What is Science and what is the so-called Scientific Method? Science is a systematic attempt to understand natural phenomena in as much detail and depth as possible, and use the knowledge so gained to predict, modify and control phenomena. Science is exploring, experimenting and predicting from what we see around us. The curiosity to learn about the world, unravelling the secrets of nature is the first step towards the discovery of science. The scientific method involves several interconnected steps: Systematic observations, controlled experiments, qualitative and quantitative reasoning, mathematical modelling. prediction and verification or falsification of theories. Speculation and conjecture also have a place in science: but ultimately, a scientific theory, to be acceptable. must be verified by relevant observations or experiments. There is much philosophical debate about the nature and method of science that we need not discuss here.
The interplay of theory and observation (or experiment) is basic to the progress of science. Science is ever dynamic. There is no ‘final’ theory in science and no unquestioned authority among scientists. As observations improve in detail and precision or experiments yield new results, theories must account for them, if necessary, by introducing modifications. Sometimes the modifications may not be drastic and may lie within the framework of existing theory. For example, when Johannes Kepler (1571-1630) examined the extensive data on planetary motion collected by Tycho Brahe (1546-1601), the planetary circular orbits in heliocentric theory (sun at the centre of the solar system) imagined by Nicolas Copernicus (1473-1543) had to be replaced by elliptical orbits to fit the data better. Occasionally. however, the existing theory is simply unable to explain new observations.
This causes a major upheaval in science. In the beginning of the twentieth century, it was realised that Newtonian mechanics, till then a very successful theory, could not explain some of the most basic features of atomic phenomena. Similarly, the then accepted wave picture of light failed to explain the photoelectric effect properly. This led to the development of a radically new theory (Quantum Mechanics) to deal with atomic and molecular phenomena.
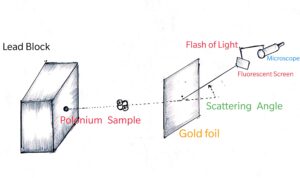
Just as a new experiment may suggest an alternative theoretical model, a theoretical advance may suggest what to look for in some experiments. The result of experiment of scattering of alpha particles by gold foil, in 1911 by Ernest Rutherford (1871-1937) established the nuclear model of the atom, which then became the basis of the quantum theory of hydrogen atom given in 1913 by Niels Bohr (1885-1962). On the other hand, the concept of antiparticle was first introduced theoretically by Paul Dirac (1902-1984) in 1960 and confirmed two years later by the experimental discovery of positron (antielectron) by Carl Anderson.
Physics is a basic discipline in the category of Natural Sciences, which also includes other disciplines like Chemistry and Biology. The word Physics comes from a Greek word meaning nature. Its Sanskrit equivalent is Bhautiki that is used to refer to the study of the physical world. A precise definition of this discipline is neither possible nor necessary. We can broadly describe physics as a study of the basic laws of nature- and their manifestation in different natural phenomena. The scope of physics ís described briefly in the next section. Here we remark on two principal thrusts in physics: unification and reduction
The In Physics, we attempt to explain diverse physical phenomena in terms of a few concepts and laws. The effort is to see the physical world as manifestation of some universal laws in different domains and conditions. For example, the same law of gravitation (given by Newton) describes the fall of an apple to the ground, the motion of the moon around the earth and the motion of planets around the sun. Similarly, the basic laws of electromagnetism (Maxwell’s equations) govern all electric and magnetic phenomena. The attempts to unify fundamental forces of nature (section 1.4) reflect this same quest for unification.
A related effort is to derive the properties of a bigger, more complex, system from the properties and interactions of its constituent simpler parts. This approach is called reductionism and is at the heart of physics. For example, the subject of thermodynamics, developed in the nineteenth century, deals with bulk systems in terms of macroscopic quantities such as temperature. internal energy, entropy, etc. Subsequently, the subjects of kinetic theory and statistical mechanics interpreted these quantities in terms of the properties of the molecular constituents of the bulk system. In particular, the temperature was seen to be related to the average kinetic energy of molecules of the system.
1.2 SCOPE AND EXCITEMENT OF PHYSICS
The scope of physics can be understood by exploring its various sub-disciplines, which are broadly categorized into two domains: macroscopic and microscopic. The macroscopic domain encompasses phenomena at laboratory, terrestrial, and astronomical scales, while the microscopic domain focuses on smaller-scale phenomena. Classical physics primarily deals with macroscopic phenomena and includes subjects like mechanics, electrodynamics, optics, and thermodynamics. Mechanics, based on Newton’s laws of motion and the law of gravitation, studies the motion and equilibrium of particles, rigid bodies, and systems of particles.
Examples include rocket propulsion by ejecting gases, wave propagation in water or air, and the equilibrium of a bent rod under a load. Electrodynamics explores electric and magnetic phenomena related to charged and magnetic bodies, with foundational laws established by Coulomb, Oersted, Ampere, and Faraday, and later unified by Maxwell’s equations. Problems in electrodynamics include the motion of a current-carrying conductor in a magnetic field, the response of circuits to alternating currents, the operation of antennas, and the propagation of radio waves through the ionosphere.
Optics deals with the phenomena involving light. The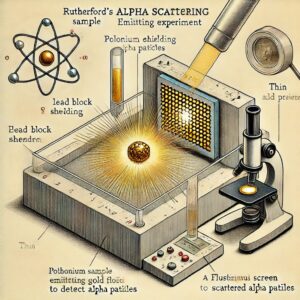 working of telescopes and microscopes, colours exhibited by thin films, etc., are topics in optics. Thermodynamics, in contrast to mechanics, does not deal with the motion of bodies as a whole. Rather, it deals with systems in macroscopic equilibrium and is concerned with changes in internal energy, temperature, entropy, etc., of the system through external work and transfer of heat. The efficiency of heat engines and refrigerators, the direction of a physical or chemical process, etc., are problems of interest in thermodynamics.
working of telescopes and microscopes, colours exhibited by thin films, etc., are topics in optics. Thermodynamics, in contrast to mechanics, does not deal with the motion of bodies as a whole. Rather, it deals with systems in macroscopic equilibrium and is concerned with changes in internal energy, temperature, entropy, etc., of the system through external work and transfer of heat. The efficiency of heat engines and refrigerators, the direction of a physical or chemical process, etc., are problems of interest in thermodynamics.
The microscopic domain of physics deals with the constitution and structure of matter at the minute scales of atoms and nuclei (and even lower scales of length) and their interaction with different probes such as electrons, photons and other elementary particles. Classical physics is inadequate to handle this domain and Quantum Theory is currently accepted as the proper framework for explaining microscopic phenomena. Overall, the edifice of physics is beautiful and imposing and you will appreciate it more as you pursue the subject.
You can now see that the scope of physics is truly vast. It covers a tremendous range of magnitude of physical quantities like length. mass, time, energy, etc. At one end, it studies phenomena at the very small scale of length (10 ^ – 14 or even less) involving electrons, protons. etc.; at the other end, it deals with astronomical phenomena at the scale of galaxies or even the entire universe whose extent is of the order of 10 ^ 26 m. The two length scales differ by a factor of 10 ^ 40 or even more. The range of time scales can be obtained by dividing the length scales by the speed of light: 10 ^ – 22 to 10 ^ 18. The range of masses goes from, say. 10 ^ – 30 kg (mass of an electron) to 10 ^ 55 kg (mass of known observable universe). Terrestrial phenomena lie somewhereé in the middle of this range.
 Physics is exciting in many ways. To some people the excitement comes from the elegance and universality of its basic theories, from the fact that a few baste concepts and laws can explain phenomena covering a large range of magnitude of physical quantities. To some others, the challenge in carrying out imaginative new experiments to unlock the secrets of nature, to verify or refute theories, is thrilling. Applied physics is equally demanding. Application and exploitation of physical laws to make useful devices is the most interesting and exciting part and requires great ingenuity and persistence of effort.
Physics is exciting in many ways. To some people the excitement comes from the elegance and universality of its basic theories, from the fact that a few baste concepts and laws can explain phenomena covering a large range of magnitude of physical quantities. To some others, the challenge in carrying out imaginative new experiments to unlock the secrets of nature, to verify or refute theories, is thrilling. Applied physics is equally demanding. Application and exploitation of physical laws to make useful devices is the most interesting and exciting part and requires great ingenuity and persistence of effort.
What lies behind the phenomenal progress of physics in the last few centuries? Great progress usually accompanies changes in our basic perceptions. First, it was realised that for scientific progress, only qualitative thinking. though no doubt important, is not enough. Quantitative measurement is central to the growth of science, especially physics, because the laws of nature happen to be expressible in precise mathematical equations.
The second most important insight was that the basic laws of physics are universal the same laws apply in widely different contexts. Lastly, the strategy of approximation turned out to be very successful. Most observed phenomena in daily life are rather complicated manifestations of the basic laws.
Scientists recognised the importance of extracting the essential features of a phenomenon from its less significant aspects. It is not practical to take into account all the complexities of a phenomenon in one go. A good strategy is to focus first on the essential features, discover the basic principles and then introduce corrections to build a more refined theory of the phenomenon. For example, a stone and a feather dropped from the same height do not reach the ground at the same time.
The reason is that the essential aspect of the phenomenon, namely free fall under gravity, is complicated by the presence of air resistance. To get the law of free fall under gravity, it is better to create a situation wherein the air resistance is negligible. We can, for example, let the stone and the feather fall through a long evacuated tube. In that case, the two objects will fall almost at the same rate, giving the basic law that acceleration due to gravity is independent of the mass of the object. With the basic law thus found, we can go back to the feather, Introduce corrections due to air resistance, modify the existing theory, and try to build a more realistic theory of objects falling to the earth under gravity.