States of Matter
Matter exists in three primary states: solid, liquid, and gas. A mineral is a naturally occurring inorganic substance with a specific chemical composition and, in most cases, a crystalline structure. While the majority of minerals are solid, some, like native mercury, are naturally liquid, and others, like natural gas, are gaseous.
Gases and liquids are collectively termed “fluids” because they flow under the influence of gravity at normal atmospheric temperature (T) and pressure (P). Solids, in contrast, generally do not flow at atmospheric conditions but can do so at much higher temperatures and pressures. A gas expands to fill its container, while a liquid may be constrained by an upper horizontal surface.
Most pure substances can exist in all three states (solid, liquid, gas) depending on the combination of temperature and pressure. At a specific temperature, known as the melting point, many minerals transition from solid to liquid, although some minerals may decompose before melting. A sublimate occurs when a gas directly condenses into a solid without passing through the liquid phase.
Elements, Compounds, and Mixtures
A pure substance has consistent and unchanging properties. Pure substances fall into two categories: elements and compounds. Elements are substances that have not been broken down into simpler substances by ordinary chemical means. Although over 100 elements are currently known, many are either rare or unstable and of limited importance to mineralogy.
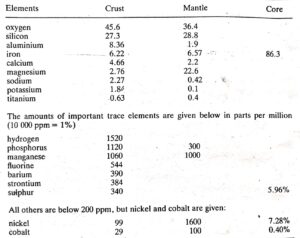
The estimated composition of the Earth’s three layers, based on the most important elements by weight, shows that just over 99% of the Earth’s crust consists of eight elements. In descending order, these elements are oxygen (O), silicon (Si), aluminum (Al), iron (Fe), calcium (Ca), magnesium (Mg), sodium (Na), and potassium (K). Notably, many economically significant elements are absent from this list
Compounds vs. Mixtures
Compounds are pure substances composed of two or more elements that are chemically bonded together. They differ from mixtures in several key ways:
- Definite Proportions: The elements in a compound are combined in fixed proportions by weight, unlike mixtures, where the components can vary in quantity.
- Difficulty in Separation: Compounds cannot be easily separated into their constituent elements, while the components of a mixture can usually be separated by physical or mechanical means. These components can be either elements or other compounds.
- Distinct Properties: The properties of a compound are often entirely different from those of the elements that make it up. In contrast, mixtures tend to retain the properties of their individual constituents.
- Heat Involvement: The formation of a compound typically involves a chemical reaction in which heat is either released (exothermic) or absorbed (endothermic). Mixing substances, on the other hand, may not involve any heat change.
Minerals and Rocks
Minerals are compounds made of specific elements, whereas rocks are mixtures of various minerals. For example, the mineral quartz (SiO₂) is a compound consisting of silicon (Si) and oxygen (O). On the other hand, granite is a rock made up of a mixture of several minerals, including quartz.
